News
We are pleased to announce that, by Resolution No. 12/IV/2025 of the Health Sciences Discipline Council of the Jagiellonian University dated December 17, 2025, Katarzyna Klaś was awarded the degree of Doctor in the discipline of Health Sciences.
In October, Tomasz completed an internship at the Amsterdam University Medical Center (Amsterdam UMC), organized as part of the project “Teaching-Engagement-Development (TED): Academic Teachers and Doctoral Students Facing the Challenges of the Future.”
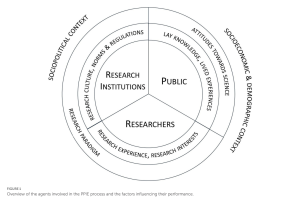
We kindly recommend to you a new paper published by the members of the Department of Bioethics and Health Psychology entitled: Meaningful public involvement: changing research institutions toward epistemic justice.
In this paper, we argue for comprehensive changes within research institutions to enable meaningful PPIE practices and propose a framework of institutional changes that focuses on their sociocultural and epistemic dimensions.
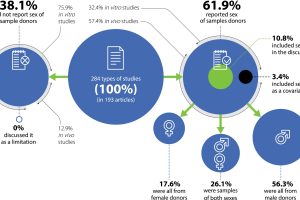
We would like to invite you to read a new article published in the JOR Spine. This article aims to provide a comprehensive overview of how donor sex is reported in intervertebral disc degeneration and osteoarthritis preclinical research using human or animal samples and in vivo models. This article is part of the CARTHAGO project, which was funded by the European Commission’s Horizon 2020 programme.
From 13 to 16 August 2025, Marcin Waligora and Tomasz Krawczyk took part in the 37th European Conference on Philosophy of Medicine and Healthcare, which had the theme of ‘Bioethics and Philosophy of Medicine after the Pandemic’. The conference took place in Manchester, UK. Marcin Waligora presented his work, ‘Direct and surrogate benefit in cancer clinical trials’, while Tomasz Krawczyk discussed ‘Dialogue towards ethical participation. A qualitative study of Deaf people’s research experiences’.